Cell therapies are treatments that use cells from the patient or a donor to repair or replace damaged cells and tissues, helping the body restore normal function or enhance its ability to fight diseases.


Science
Autoimmune diseases, such as lupus and myasthenia gravis, occur when the immune system mistakenly attacks the body.
There are more than
80
Autoimmune diseases
Impacting approximately
10%
of the global population
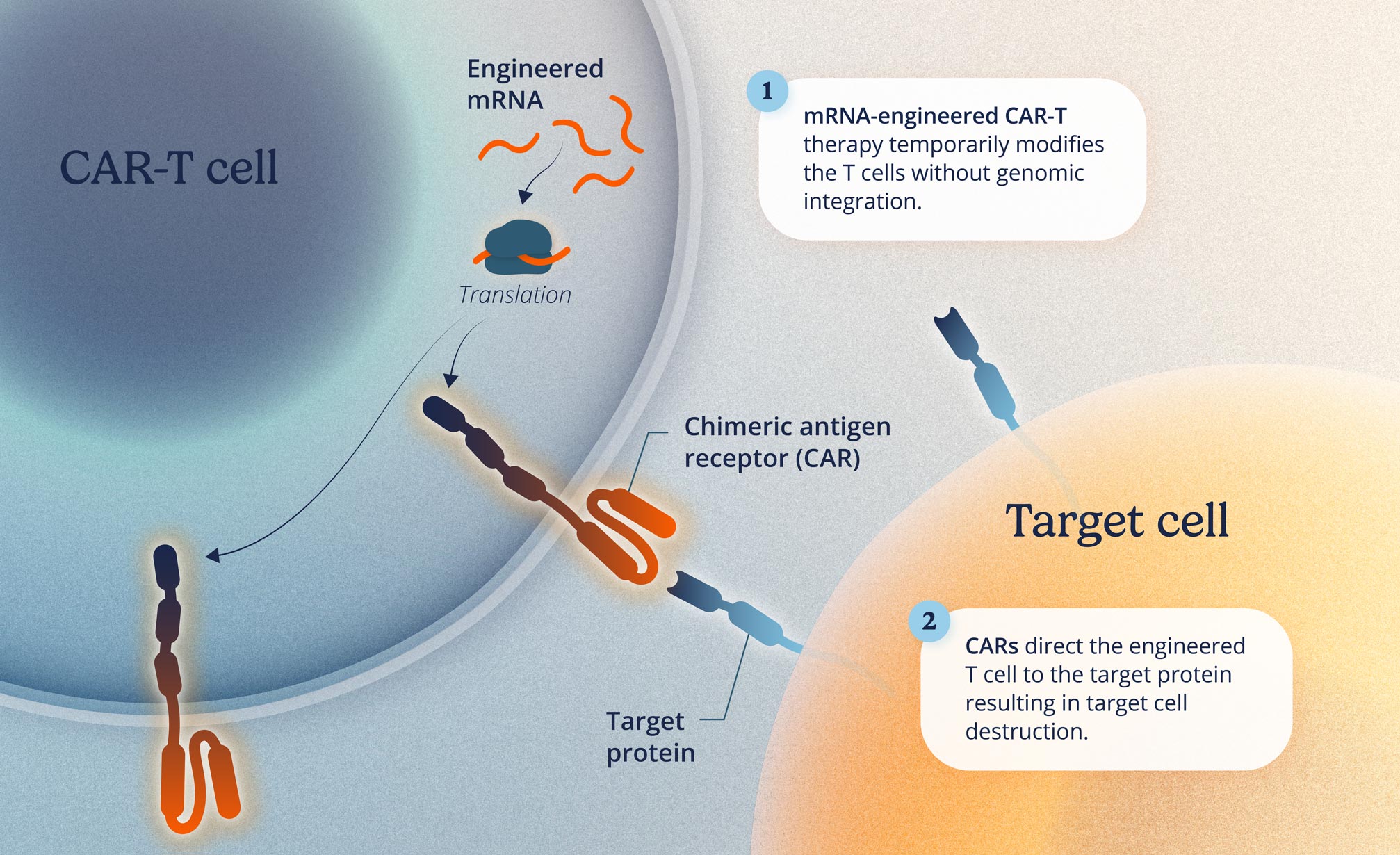
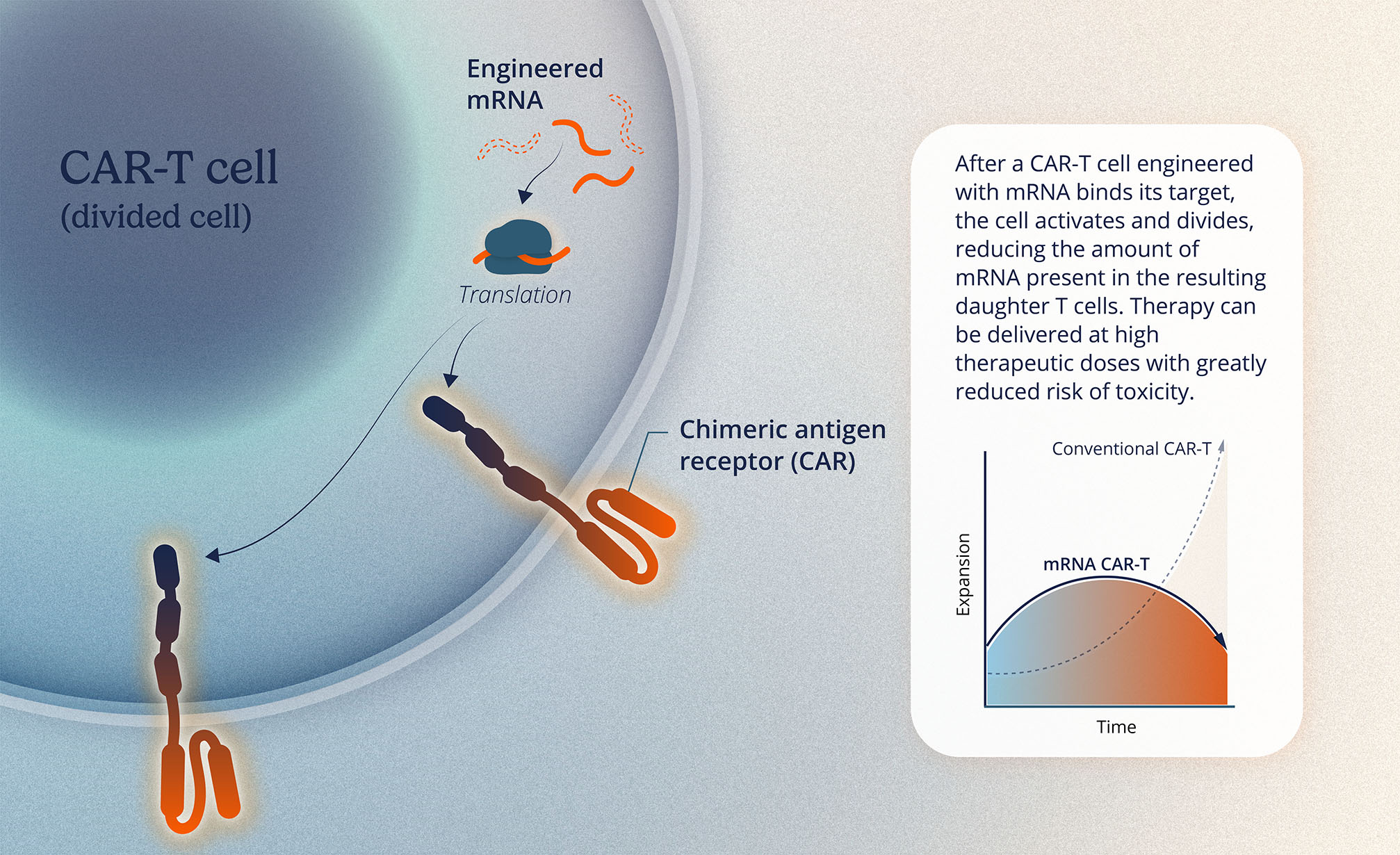
Current cell therapies have been helpful in treating certain types of cancer, but the application had not been used for autoimmune diseases. Current treatments require patients to undergo chemotherapy before starting, typically performed in a hospital. This can lead to challenges related to side effects, complexity during treatment, and high costs.
Manufacturing

Publications & Presentations