Pipeline

Cartesian is advancing its pipeline of mRNA cell therapies, which includes multiple clinical assets, for the treatment of autoimmune conditions. Cartesian is also pursuing new ideas in its discovery programs.
- * Includes juvenile dermatomyositis in addition to adult myositis indications

Descartes-08
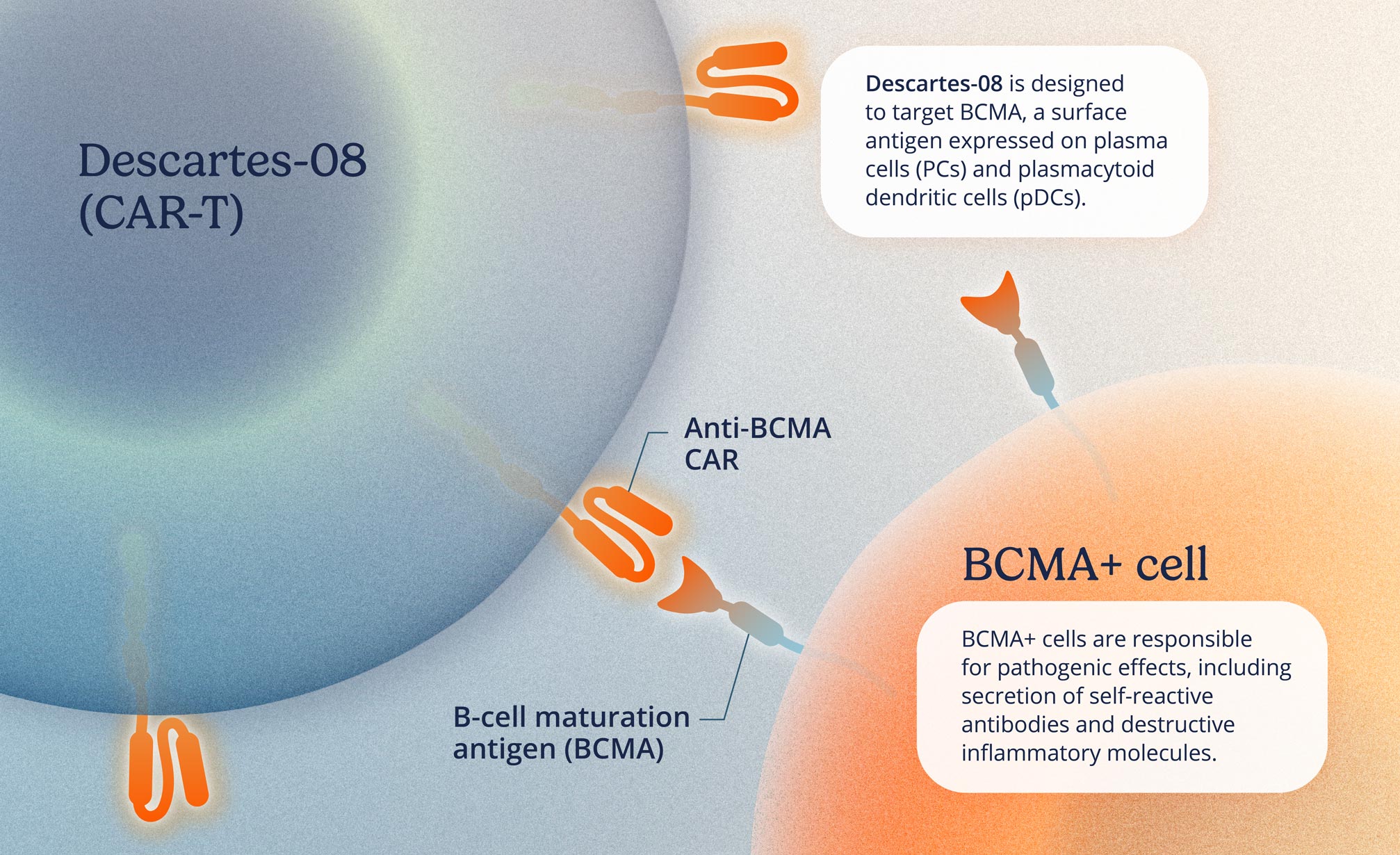
Descartes-08, Cartesian’s lead candidate, is an mRNA chimeric antigen receptor T-cell cell therapy (mRNA CAR-T) in clinical development for autoimmune disease. CAR-T cell therapy involves modifying a patient’s T cells—key components of the immune system that help identify and attack pathogens—to selectively target and suppress the immune responses that are believed to contribute to their condition.
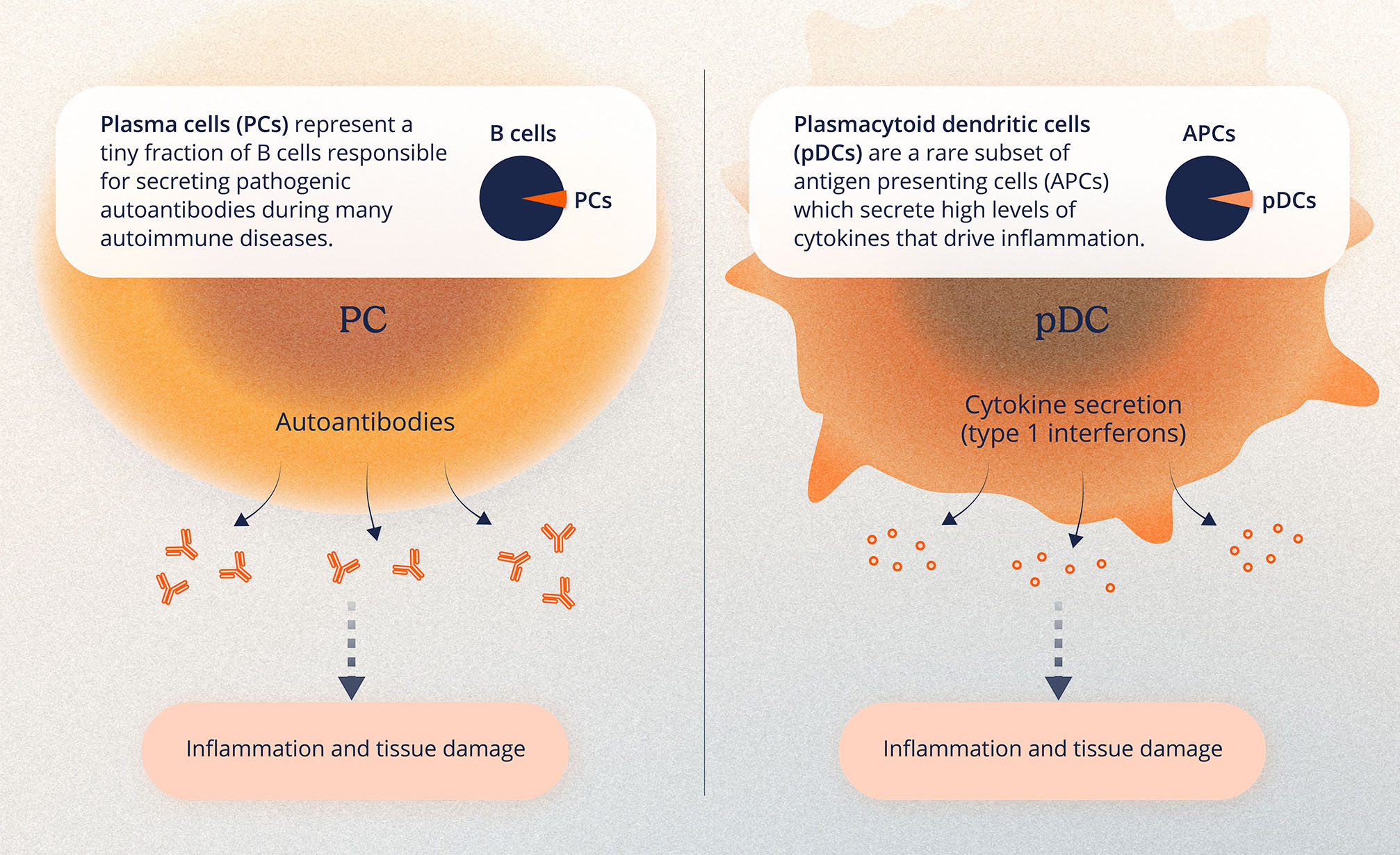
Descartes-08 is an autologous mRNA CAR-T, which means it uses a patient’s own T cells modified with mRNA to help them better target and fight their disease. More specifically, Descartes-08 targets B-cell maturation antigen (BCMA), a cell surface protein involved in regulating immune responses, to help modulate the overactive immune activity seen in certain autoimmune diseases.
Descartes-08 is designed to be dosed safely in an outpatient setting without pretreatment chemotherapy. In a Phase 2 clinical trial in patients with generalized myasthenia gravis (MG), Descartes-08 was observed to be well tolerated, and adverse events were transient and mostly mild, supporting outpatient administration without the need for pretreatment chemotherapy.
Cartesian is developing Descartes-08 for the treatment of generalized MG and myositis.
Cartesian has been awarded several grants related to Descartes-08 from the National Institutes of Health (NIH) and is working with the NIH to advance the development of Descartes-08 through a research partnership. Descartes-08 has been granted Regenerative Medicine Advanced Therapy Designation and Orphan Drug Designation for the treatment of MG, as well as Rare Pediatric Disease Designation for the treatment of JDM, by the U.S. Food and Drug Administration.
These FDA-sponsored designations support Cartesian, offering benefits at different stages throughout clinical development and the potential approval process. For example, receiving RMAT Designation offers sponsor companies the benefits of the fast track and breakthrough therapy designation programs, allowing for early, close, and frequent interactions with the FDA with the goal of expediting drug development.

Descartes-15
Descartes-15 is Cartesian’s next-generation autologous B cell maturation antigen (BCMA)-directed mRNA-engineered chimeric antigen receptor T-cell therapy (mRNA CAR-T). Descartes-15 is designed to have predictable and controllable pharmacokinetics, including technological advances that enhance CAR stability even in the presence of target-driven suppression of CAR.
Similar to Descartes-08, the Company’s lead product candidate, Descartes-15 is designed to be administered without preconditioning chemotherapy and does not use integrating vectors. Relative to Descartes-08, Descartes-15 has been observed to achieve an approximately ten-fold increase in CAR expression and selective target-specific killing in preclinical studies.
A Phase 1 dose escalation trial (NCT04816526) demonstrated favorable safety and tolerability data in participants treated with Descartes-15. Cartesian plans to pause the development of Descartes-15 to prioritize later-stage opportunities with Descartes-08 in MG and myositis.